MEDICAL DEVICE AND IVD CONSULTING
Great work in highly regulated environment...
...starts with knowledge and passion!
Contact us...starts with knowledge and passion!
▼

OUR PROFILE
mdXperts is based in Wernigerode, Germany and Dublin, Ireland. mdXperts is a regulatory consultancy providing analytical and strategic services to the international medical devices and medical technology market. We support manufacturers and economic operators in the medical device life cycle management, the medical device approval and clearance process in Europe (European Union, Switzerland, und United Kingdom), international product registration and in the development of quality management systems.
mdXperts provides services in the area of medical device life cycle from the product concept via development to the post market period. mdXperts supports manufacturer in devices development in a design control regime (design control, medical device risk management, human factor engineering) to ensure that resulting medical device are comprehensive verified and validated and meet objectively the European und US American regulatory requirements.
mdXperts was founded in 2021 by Karin Brueckmann Mercurio MDC, Dublin and Uwe Wallstab, Engineering Office for Medical Technology, Cologne to combine expertise under one international brand. Thus, mdXperts stands for more than twenty years of consulting know-how in the highly regulated field of medical technology. The mdXperts group is located in Europe and includes the mdXperts GmbH,
a company under German law with the main office in Wernigerode, Germany and mdXperts - Mercurio -, Dublin Ireland.

The focus of my work is on medical device life cycle, in particular risk management, human factor engineering, design control, validation, and conformity assessment, with 20 years of experience.
Specialization
Uwe has particular experience in technologies for extracorporeal blood circulation and blood treatment.

I have been working as a self-employed QA / RA Consultant since 2017, before which I worked as Regulatory Affairs Officer and QA/RA Project Project Manager .
Specialization
Karin's main focus lies in international market clearance, product registration, compiling submission dossiers and project control.

MANAGEMENT PARTNERS
Our team consists of multilingual experts - biomedical engineers, medical doctors, chemists and regulatory affairs experts- with an extensive medical, scientific, regulatory, technical and business background who are well-respected by international notified bodies and competent authorities. We specialize in the fields of medical technology and regulatory affairs. We have profound knowledge in the areas of quality management, USA and European regulatory requirements, EU conformity assessment process (CE marking), product safety, planning and performance of production process, product validation and clinical evaluation.
The objective of mdXperts is to provide excellent and hands-on assistance for our clients in an efficient and flexible way. Considering the client’s perspectives, we tailor our competent services to meet the customer‘s needs in a cost-effective manner and within a clear timeline. We understand and enjoy the entrepreneurial spirit inherent in the small and medium sized companies. Benefitting from years of experience in the fields of medical device development, production control, quality assurance and regulatory affairs, mdXperts offers full-service packages in order to speed up the process from product development to market clearance. Our commitment is to help our clients to obtain a remarkable advantage in their target markets.

OUR TEAM AND PHILOSOPHY
Having access to a trusted global network of partners in the United Kingdom, Switzerland, Australia, Israel, Japan, Canada and USA, mdXperts is a key service provider offering in-depth knowledge across the lifecycle of medical technology from product development to the post-market period in key medical devices markets.
mdXperts as part of the MDR COMPETENCE network has direct access to a network of specialized consultants, and can, in cooperation with accredited test houses, support you in the planning and execution of even complex verification and validation activities in the field of chemical characterization, biological safety, packaging, transport and storage validation as well as electical safety and electromagnetic compatibility testing.
mdXperts has comprehensive expertise in the design and standard-compliant development of medical electrical equipment in accordance with IEC 60601-x and numerous particular standards for corresponding equipment groups. We have extensive experience in the implementation of customer projects in cooperation with development service providers.

OUR NETWORK
mdXperts has in-depth knowledge of extracorporeal blood treatment methods, such as chronic hemodialysis, continuous extracorporeal renal replacement therapy, therapeutic apheresis and extracorporeal liver support. We are familiar with the technologies of infusion pumps and volumetric infusion controllers and cardiopulmonary extracorporeal circulation. Our work focuses on the required technologies, essential performance characteristics, system and safety architecture, and functional safety of extracorporeal blood treatment systems.
Expertise in the field of Extracorporeal blood circulation & blood treatment
Extracorporeal renal replacement therapy technologies
Extracorporeal liver support technologies
Cardiopulmonary bypass machine (HLM)
Extracorporeal cardiopulmonary support (ECMO)
Therapeutic apheresis
Expertise in the field of Fluid management and water treatment
Reverse osmosis technology
Dialysis fluids and concentrates
Infusion pump technologies / volumetric infusion controller
Filtration technologies
Adsorption technologies
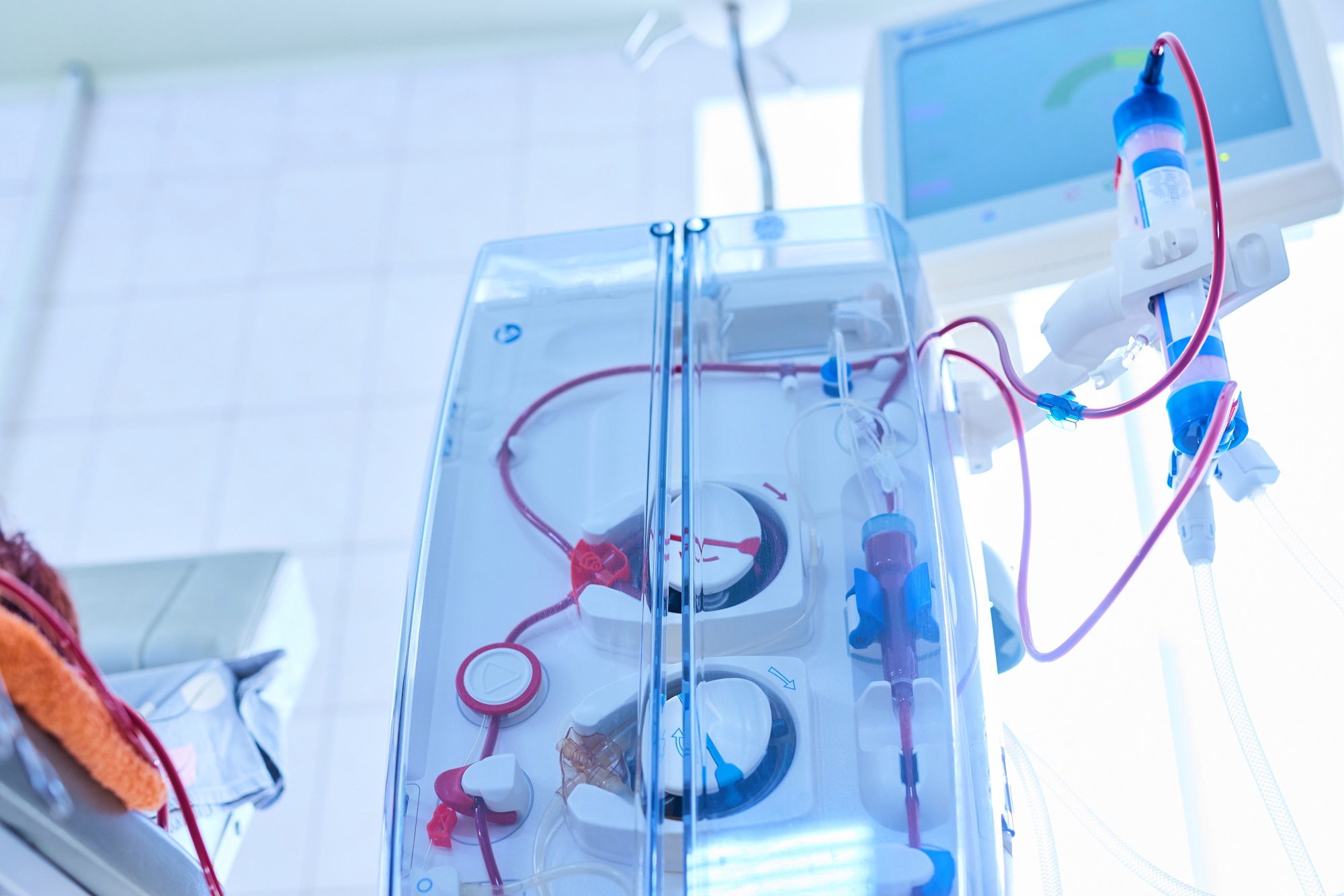
PARTICULAR TECHNOLOGY KNOWLEDGE
Please contact any of the associates at:
Germany
mdXperts GmbH
IGZ - Innovation Center
Office Unit A 15
Dornbergsweg 2
38855 Wernigerode
Ireland
Mercurio - mdXperts Ltd.
13 Adelaide Road,
Saint Peter’s, Dublin
D02 P950
Saint Peter’s, Dublin
D02 P950



